Assessment of pre-transplant renal function as measured by estimated Glomerular Filtration Rate (eGFR) is a standard criterion for selection of patients for allogeneic hematopoietic cell transplantation (alloHCT). Despite refinements in alloHCT and reductions in toxicity over time, we hypothesized that baseline renal function is still independently associated with differences in outcomes in the contemporary era. The aim of this study was to identify an optimal eGFR cut point for identifying those at increased risk of HCT-related mortality.
Patients who underwent first alloHCT from January 2012 to December 2021 were identified from our institutional database, excluding syngeneic and umbilical cord grafts. eGFR was calculated using the CKD-EPI Creatinine Equation (Inker et a. NEJM 2021). Optimal pre-HCT eGFR cut point for high-risk of non-relapse mortality (NRM) after alloHCT was identified using Cox restricted cubic spline plot. eGRF was grouped into multiple categories based upon the observed association in the spline plot alone with an established cut point of 90 ml/min/1.73 m² for abnormal kidney function. Associations of eGFR, demographics, prognostic factors, and HCT characteristics with NRM and overall survival (OS) were evaluated using survival analysis; non-HCT related death was considered as a competing risk in the NRM analysis.
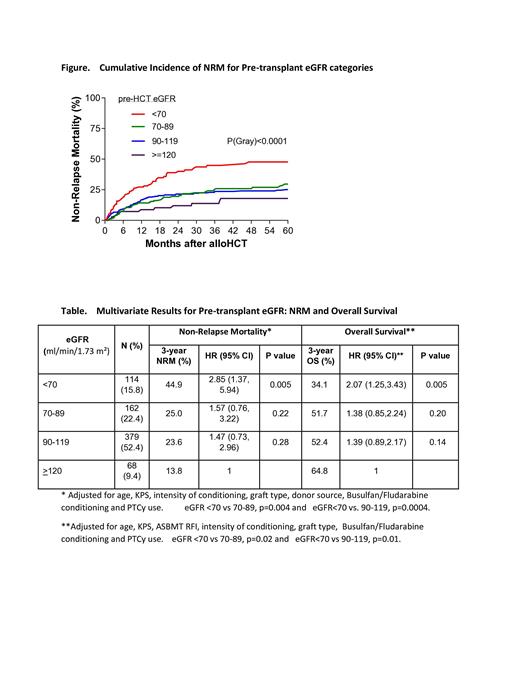
724 adult patients were studied with a median age of 58 yrs (range, 18-79), 57% males, 92% Caucasian, and 76% KPS >90. HCT-CI: 52% high, 31% intermediate, and 17% low risk. Transplants included 43% myeloablative and 57% reduced-intensity conditioning; 64% peripheral blood and 36% bone marrow grafts. Donor type: 28% related, 55% unrelated and 17% haploidentical. The median (IQR) of pre-transplant eGFR was 96.7 ml/min/1.73 m² (79.6, 107.5). eGFR was categorized as <70 (15.8%), 70-89 (22.4%), 90-119 (52.4%), and >120 ml/min/1.73 m² (9.4%). NRM was significantly higher for the eGFR <70 ml/min/1.73 m² group compared to the others (3-year NRM: 44.9% in eGFR <70 vs 25.0% in 70-89, 23.6% in 90-119, and 13.8% in >120 ml/min/1.73 m²) which was confirmed on multivariate analysis (Figure and Table). Overall survival (OS) was also significantly worse for those with an eGFR <70 ml/min/1.73 m² on multivariate analysis (3-year OS: 34.1% in eGFR <70 vs 51.7% in 70-89, 52.4% in 90-119, and 64.8% in >120 ml/min/1.73 m²) (Table) along with baseline KPS and high ASBMT Request for Information (RFI). The most common causes of death for both the eGFR<70 and >70 ml/min/1.73 m² groups were relapse (44% vs 60%, respectively) and infection (25% vs. 19%, respectively). Both NRM and OS differences among the 3 higher eGFR groups were not statistically significant in multivariate analyses. eGFR was not associated with relapse.
We conclude that pre-transplant eGFR <70 ml/min/1.73m 2 is an independent prognostic factor for mortality after alloHCT. Future investigation with larger subgroup analyses regarding specific diagnoses, conditioning regimens and transplant modalities may further identify which patients are more appropriate for transplant and those who may benefit from alternative treatment approaches. Our results also suggest that additional efforts to preserve renal function prior to transplant by limiting nephrotoxic exposures may have implications for optimizing outcomes after transplant, particularly in those with other comorbidities.
Disclosures
Sobecks:CareDx: Membership on an entity's Board of Directors or advisory committees. Hanna:SOBI: Speakers Bureau; Sanofi: Speakers Bureau; EDITAS: Research Funding; Vertex: Honoraria. Rotz:RCI BMT: Other: medical monitor for NMDP. Jagadeesh:AstraZeneca: Research Funding; Trillium Pharmaceuticals: Research Funding; Debio Pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Regeneron Pharmaceuticals: Research Funding; Seagen: Research Funding; ATARA Biotherapeutics: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Gerds:Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding; AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy. Hamilton:Equilium: Other: ad hoc advisory board; Kadmon/Sanofi: Other: advisory board; NKARTA: Other: ad hoc advisory board; Angiocrine: Other: DSMB; Therakos: Honoraria; Incyte: Other: ad hoc consultancy; Rigel: Other: Ad hoc advisory board; CSL Behring: Other: Adjudication committee. Brunstein:Allovir DSMB: Other: Consultant. Sauter:Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK.: Consultancy; Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal